
Japanese iPSC On the Cusp of a Stem Cell Revolution: Research Turns to Clinical Applications
Science Technology- English
- 日本語
- 简体字
- 繁體字
- Français
- Español
- العربية
- Русский
Ever since Nobel laureate Yamanaka Shin’ya created the first human induced pluripotent stem cells (iPS cells, or iPSC) in 2006, the medical community has been abuzz with talk of the technology’s therapeutic potential. Using Yamanaka’s technique, cells from a tiny sample of skin or blood can be reprogrammed and cultured to create large numbers of stem cells capable of differentiating into the specialized cells that make up such organs as muscle, bone, heart, liver, blood vessels, and nerves.
While much of the excitement surrounding clinical applications has centered on regenerative medicine, Yamanaka, who directs Kyoto University’s Center for iPS Cell Research and Application (CiRA), was quick to recognize the potential role of iPS cells in drug development. In September this year, CiRA reached a major milestone with the launch of the world’s first clinical trial for a drug candidate identified through iPSC study and screening.
Hope for Victims of Rare Diseases
Led by CiRA Deputy Director Toguchida Jun’ya, the trials will test the efficacy of an existing drug, already approved for other purposes, in the treatment of fibrodysplasia ossificans progressiva, or FOP. FOP is a rare and serious genetic disorder in which the body’s connective tissues—muscles, tendons, and ligaments—progressively harden into bone. This ossification, which begins during childhood, causes loss of mobility, as well as eating and breathing difficulties that ultimately lead to death. Although scientists have pinpointed the genetic mutation responsible, there is currently no cure or approved treatment.
Affecting just one in 2 million people worldwide, and about 80 patients in Japan today, FOP is among the designated intractable diseases eligible for Japanese government funding under a special system to support study and treatment of rare diseases.
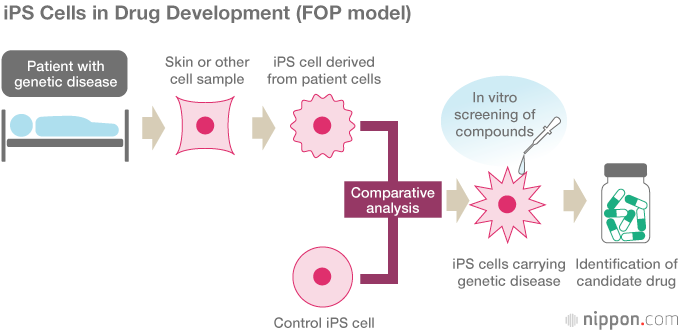
Toguchida’s team began by successfully reprogramming iPS cells from the cells of FOP patients. Comparing them with control iPS cells free from the FOP mutation, the researchers found that the FOP cells exhibited a much higher capacity for bone formation and mineral deposition in vitro, and they were able to identify key pathways in the mechanism of abnormal bone formation. In this way, they created a “disease model in a dish” without the use of animal models. Using the iPS cells to screen some 6,800 compounds for their effect, they found that the immunosuppressive drug rapamycin inhibited abnormal bone formation.
Trials of rapamycin as a treatment for FOP will be conducted at the hospitals attached to Kyoto, Kyushu, and Nagoya Universities and the University of Tokyo. Twenty patients aged six and up will be divided into two groups, with one group receiving rapamycin over a period of six months.
Rapamycin is currently used in Europe to prevent rejection of transplanted organs. In Japan, it was approved in 2014 for the treatment of lymphangioleiomyomatosis, another rare and intractable disease. The identification of new applications for rapamycin is an example of “drug repositioning.” Repurposing a drug that has already been tested and approved greatly reduces the risk that serious side effects will emerge during clinical testing. It is thus far less costly than the conventional discovery and development process, since safety and pharmacokinetic testing on human subjects has already been completed.
Even so, clinical trials cost in the tens of millions of yen, and in the case of a rare condition like FOP, the market is far too small to attract the commercial interest of pharmaceutical companies. This is why the government now funds research and clinical trials to test existing drugs for the treatment of rare and intractable diseases.
Toward a New Era in Drug Development
Another example of the use of iPS cells derived from patients with specific diseases (disease-specific iPSCs) is the clinical research on amyotrophic lateral sclerosis (ALS) led by Professor Okano Hideyuki of Keiō University. Using iPSC-based screening, Okano’s team identified a candidate drug for treatment of this fatal disease, which progressively destroys the body’s motor nerve cells until patients are unable to move, speak, or breathe. Clinical trials are expected to begin in 2018.
Researchers working under Okano also discovered that rapamycin can inhibit destruction of inner-ear cells in Pendred’s syndrome, a common cause of hereditary hearing loss. And a team working under Keiō University Professor Fukuda Keiichi found that a drug currently used to treat pulmonary arterial hypertension holds promise for treatment for hypertrophic cardiomyopathy, a hereditary thickening of the heart muscle leading to heart failure.
Back at CiRA, Professor Tsumaki Noriyuki’s research team has used iPSC technology to identify a candidate drug for treatment of two rare and intractable diseases of the skeletal system. They found that statins, already used worldwide to lower cholesterol levels, had a positive effect on cartilage and bone formation in achondroplasia, a cause of dwarfism, and thanatophoric dysplasia, a lethal skeletal disorder.
Horizons in Regenerative Medicine
The other major potential clinical application for iPS cells is regenerative medicine: the use of stem cells to replace or regenerate damaged tissue. Since 2013, Dr. Takahashi Masayo of the Riken Center for Developmental Biology has been leading a clinical study on the use of retinal neurons differentiated from iPS cells to treat age-related macular degeneration, a common cause of blindness among the elderly. Meanwhile, in a joint project with medical equipment manufacturer Terumo, Osaka University Professor Sawa Yoshiki (president of the Japanese Society for Regenerative Medicine) and his team have been exploring the treatment of patients with advanced heart failure by grafting cell sheets made of iPSC-derived cardiac myocytes (muscle cells). Clinical trials are expected to begin soon.
Keiō University’s Okano (leader of the ALS drug-development team) is gearing up for clinical trials of a new procedure for safely transplanting iPSC-derived neural stem/progenitor cells into patients with spinal cord injuries. And in August this year, a team led by Professor Takahashi Jun of CiRA reported that monkeys showed a significant decrease in tremors and other Parkinson’s disease symptoms after receiving transplants of neurons prepared from human iPS cells. Confirmation of a treatment’s safety and efficacy in primate subjects is generally the last step before human clinical trials, which Takahashi hopes to begin sometime in 2018.
The holy grail of iPSC-based regenerative medicine is the creation of entire human organs for transplant. Sawa predicts that within the next three decades medical science will have the ability to grow human hearts, which function by the relatively simple mechanism of muscle contraction.
In 2013, CiRA began building and storing stocks of iPS cells for use in regenerative medicine. The iPS cells are reprogrammed from the cells of donor tissue classified according to human leukocyte antigen (HLA) type to minimize immunological rejection. By the end of fiscal 2017, the center will have accumulated enough different iPS cell lines to cover 30% of the Japanese population. The bank has already supplied cells to the research teams involved in the abovementioned work on age-related macular degeneration and the use of cell sheets to regenerate heart tissue.
The widespread use of iPS cells in regenerative medicine still faces major hurdles, including differentiation into the full range of human tissue, improved cell quality, and above all, prevention of tumor growth from transplanted cells. Drawing on the seminal achievements of Yamanaka and other pioneering scientists in the field, clinical researchers around Japan are working to meet these challenges and unlock the seemingly boundless potential of iPS cell technology.
(Originally published in Japanese October 12, 2017. Banner photo: Osaka University Professor Sawa Yoshiki announces submission of a plan for a clinical study on the use of iPSC-derived cells to treat heart failure, July 21, 2017. ©Jiji.)