
The Nobel Discovery Protecting 300 Million People from the Risk of Blindness
Science Technology- English
- 日本語
- 简体字
- 繁體字
- Français
- Español
- العربية
- Русский
Fighting Disease with Microbes
A microbe discovered by scientist Ōmura Satoshi in Japanese soil is helping to free 300 million people around the world from the risk of blindness. For this contribution to humankind, Ōmura was awarded one quarter of the 2015 Nobel Prize in Physiology or Medicine.
Onchocerciasis, or river blindness, is a disease that is endemic in many parts of Africa. Reflecting on the people he met who have lost their vision through the disease, Ōmura showed his happiness that the substance he found and the drug derived from it is making such a difference, while modestly stating that the microbes did all the work.
Inspired by Fermentation
Ōmura was born in 1935 as the eldest son of a farming family in Nirasaki, Yamanashi Prefecture. His father agreed to his wish to attend college, and he learned more about chemistry at the University of Yamanashi. Following graduation, he taught at an evening high school while continuing his studies at the Graduate School of the Tokyo University of Science. He became absorbed with experiments in organic chemistry, receiving a master’s degree after five years and then became a research associate in fermentation production at the Faculty of Engineering of the University of Yamanashi.
It was here that Ōmura awoke to the potential of microbes. Yamanashi Prefecture is home to many wineries, and wine-making is a major research subject at the University of Yamanashi. Impressed by how yeast can convert glucose into alcohol overnight through fermentation, Ōmura decided to do advanced research by combining the power of microbes with his knowledge of chemistry.
Wishing to devote himself to research, in 1965 Ōmura joined the Kitasato Institute, which was founded by Kitasato Shibasaburō, the father of bacteriology in Japan. He was hired as an assistant technician, a position usually assigned to college graduates. As he made clean copies of the papers written by the institute director, he built up specialist knowledge, and gained the confidence of his colleagues.
Working with Pharmaceutical Companies
In 1971, Ōmura became a visiting professor at Wesleyan University in the United States. He was invited to join the university by Max Tishler, president of the American Chemical Society and formerly the president of Merck Sharp and Dohme Research Laboratories. Here Ōmura was able to freely engage in research, and he developed friendships with leading researchers. He was, however, called back to Japan after 18 months in the United States. Research funding in Japan at that time was about one-twentieth of the level in the United States. Ōmura worked hard to raise research funds he could use after returning to Japan.
Ōmura took the approach of proposing joint research with pharmaceutical companies. The Kitasato Institute would investigate microbes and the substances they produce and when biologically active substances of interest were found through in vitro study, they would be patented and sent to a pharmaceutical company. The pharmaceutical company would perform animal testing and clinical trials. If the substance could be made into a drug and brought to market, the pharmaceutical company would pay a royalty to the institute.
This was a time when collaboration between industry and academia was viewed skeptically as a one-sided affair favoring industry. Ōmura argued, however, that finding useful drugs required collaboration with companies. Showing great foresight, he focused on developing drugs for animals rather than for people. He understood that his small group would have little chance competing against giant global corporations engaged in the heated search for human medicine. Ōmura succeeded in gaining funding from Merck, Pfizer, and other well-known pharmaceutical companies.
A Golf Course Discovery
On returning to Japan in 1973, Ōmura became the head of antibiotic research at the Kitasato Institute. Members of his team were assigned the task of collecting a spoonful of soil on their way to work or on business trips. In 1974, soil collected near a golf course on the Izu Peninsula 100 kilometers southwest of Tokyo yielded a novel microbe, Streptomyces avermectinius, which Ōmura sent to Merck. Fluid from a culture of the bacterium injected in mice infected with parasitic nematodes was found to dramatically reduce their number. This substance was named avermectin.
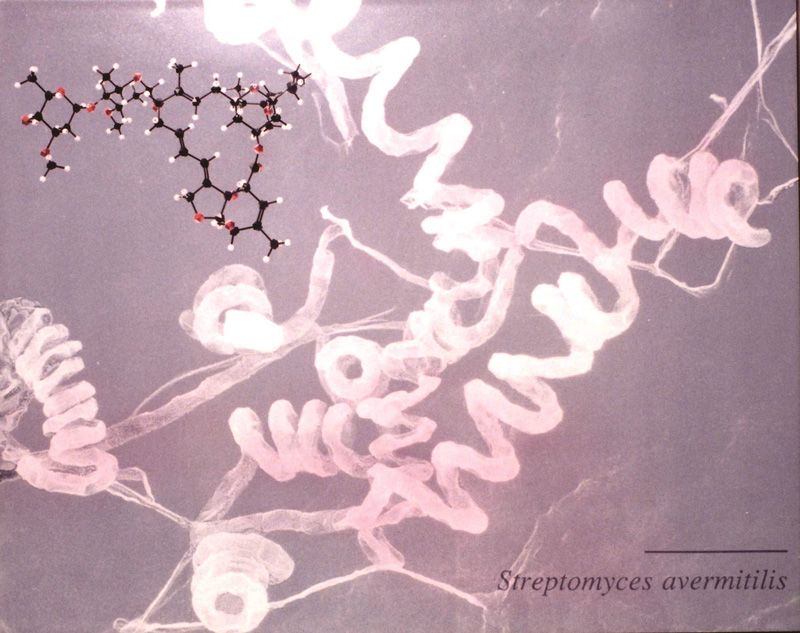 Bacteria producing avermectin found in soil at Kawana, Shizuoka Prefecture (photograph provided by the Kitasato Institute)
Bacteria producing avermectin found in soil at Kawana, Shizuoka Prefecture (photograph provided by the Kitasato Institute)
Avermectin was highly effective against parasites living in the intestinal tracts of cows and horses, almost completely eliminating them. Efforts by Merck’s synthetics group—including William C. Campbell, who shared the Nobel—to improve on avermectin led to the synthesis of ivermectin, which was launched in 1981 as Ivomec, an anti-parasitic drug for livestock and pets. Feed conversion efficiency increases greatly when nematodes living in the digestive tracts of livestock are eliminated so Ivomec found a ready market and became the top-selling animal drug in 1983. The drug contributed to a worldwide increase in food and leather production, and it was also given to pet dogs to prevent filariasis.
Effective Against Human Diseases
While the animal applications of avermectin were already a boon for humankind, the drug was also found to be effective against human diseases. Onchocerciasis is a tropical disease affecting many people in Africa that impairs the skin and eyes. The disease is spread from person to person through blackflies carrying the microfilariae (embryonic larvae) of nematodes. The microfilariae grow into adult worms in humans and release large numbers of new microfilariae, which lodge under the skin and cause intense inflammation when they die. One in five people with onchocerciasis lose their eyesight. The disease is the second leading cause of blindness after trachoma in Asia and Africa.
Ivermectin is effective only against microfilariae and not against the adult worm. This is actually a benefit since the sudden death of the adult worm can cause anaphylaxis, or an acute systemic allergic reaction, in the human host. Adult nematodes can live for 14 years in the human host, and people who are infected need to take the drug once a year during this period. The elimination of microfilariae, however, will prevent the disease from spreading to new people. This means the number of people infected with adult worms will decrease, and the disease will eventually be eradicated.
France became the first nation to approve ivermectin under the brand name Mectizan in 1987, and Merck began to provide the drug freely through the World Health Organization in 1988. Ivermectin was also found to be effective against lymphatic filariasis, a disease spread by mosquitoes where nematodes interfere with the functioning of the lymphatic system. In 2012, ivermectin was taken by more than 300 million people, in most cases distributed freely through projects to eradicate tropical diseases.
Onchocerciasis is expected to be eradicated in 2025 and lymphatic filariasis in 2020. This prospect was acclaimed by UNESCO as representing “one of the most triumphant public health campaigns ever waged in the developing world.”
A Distinctive Approach to Research
Ōmura’s research projects proved beneficial to the Kitasato Institute as its finances were in shambles around the time that joint research began, and Ōmura’s research lab was at risk of being shuttered. As Ōmura visited major pharmaceutical companies around the world, he was impressed by how many people knew and respected Kitasato Shibasaburō. Through these interactions, he came to see protecting the institute as his mission.
With Merck distributing ivermectin free of charge for human use, the institute earned royalties only on animal use. Even so, revenues from this one drug came to more than ¥22 billion, and the institute’s finances were quickly returned to a sound footing. The achievements of the Ōmura group were not limited to ivermectin. The group identified nearly 500 compounds created by microbes, of which 26 are being used as pharmaceuticals, agrochemicals, and reagents.
These achievements were supported by a distinctive approach to research. It is not easy to look for substances with a specific biological activity. Ōmura reversed this process by first discovering new compounds and then investigating their biological activity. One group worked at isolating the thousands of microbes living in one gram of soil and at identifying the functions they may have. Another group was concerned with chemical synthesis. Ōmura’s team exceeded 100 people, including students. There is little that compares to their systematic approach to discovering new substances with biological activity.
Kitasato Shibasaburō, who Ōmura highly reveres, is said to have been a candidate for the first Nobel Prize awarded in 1901. A century later, Ōmura won the Nobel Prize himself for his achievements at the institute founded by Kitasato that he worked to rehabilitate. Through the work of Ōmura, Kitasato’s desire to conquer infectious diseases has come to fruition.
(Originally written in Japanese and published on October 13, 2015. Banner photo: Ōmura Satoshi (right) being greeted with applause as he enters the Kitasato Institute the day after being awarded the Nobel Prize in Physiology or Medicine © Jiji.)
parasite ivermectin Omura Satoshi Nobel Prize in Physiology or Medicine infectious disease tropical disease antibiotic Kitasato Shibasaburō Kitasato Institute