
Will Methane Hydrates Become a Domestic Energy Resource?
Economy Science Technology- English
- 日本語
- 简体字
- 繁體字
- Français
- Español
- العربية
- Русский
On April 1, 2013, the Japanese government’s Headquarters for Ocean Policy announced draft guidelines that would steer marine strategy over the next five years. One item drew particular attention: methane hydrates. The new Basic Plan on Ocean Policy calls for assessing the extent of methane hydrate deposits surrounding Japan while simultaneously developing the necessary technology for commercially viable production of gas from this potential energy resource.(*1)
Interest is growing following the first successful extraction of methane gas from sub-seafloor hydrate deposits, which took place on March 12 in the Nankai Trough, offshore of Aichi Prefecture. However, the prospects for methane hydrates are somewhat unclear. The media is touting them as a game-changing domestic resource, while others have dismissed them as worthless.
Below I use information I have personally compiled to clarify the actual potential of methane hydrates as an energy resource.
Methane Hydrates Versus Conventional Natural Gas
The mainstream scientific view is that conventional marine reservoirs of natural gas, the primary constituent of which is methane, form in high-temperature and -pressure environments within the earth’s crust from plankton and other marine organic matter. Methane hydrates, on the other hand, form on either the sea floor or at relatively shallow burial depths in low-temperature environments.
The graph below shows the stability zone of methane hydrates. Free methane is easily produced through decomposition of organic matter by anaerobic bacteria or high temperate/pressure reactions, and water is of course readily available (even within the earth’s crust). Under conditions characterized by the lower left (shaded) portion of the graph, they may combine to form hydrates, with individual methane molecules surrounded by a “cage” of water molecules.
Marine Methane Hydrate Stability Zone
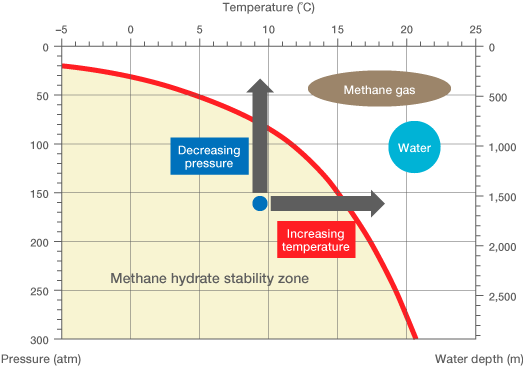
Source: Research Consortium for Methane Hydrate Resources in Japan
These conditions could be met in as little as 500 m of water, where the temperature is often below 5˚C. Therefore, it is predicted that the marine reservoir of methane contained within hydrates may be much larger than that of conventional gas. On land, however, methane hydrates are much less common, being discovered only under permafrost in locations like Canada and Russia.
Years of research have shown that significant methane hydrate deposits are present in the seafloor surrounding Japan. It is highly probable that they also exist along the seaward margins of continental shelves worldwide. In fact, the Japanese archipelago’s position along the eastern edge of the wide Eurasian continental shelf results in abundant deep environments where large amounts of methane hydrate are likely stored.
However, while the media today often cite the amount of methane hydrate in the waters surrounding Japan as equivalent to 100 years’ consumption of conventional natural gas, this amount is based on old data from a 1996 study and is probably unreliable. At present, the scope of the confirmed deposits is much more limited. Official estimates from the Nankai Trough trials, for instance, place reserves in that area at 11 times the amount of liquid natural gas imported in 2011.
Production Faces Technical Challenges
Though methane hydrate is probably widely distributed in the ocean, extraction of just the methane for use as an energy resource is extremely complicated, resulting in remarkably slow development. Once extracted and isolated, the methane can be processed and used in the exact same way as that contained within conventional gas, as well as within shale gas, which has recently seen considerable economic success.
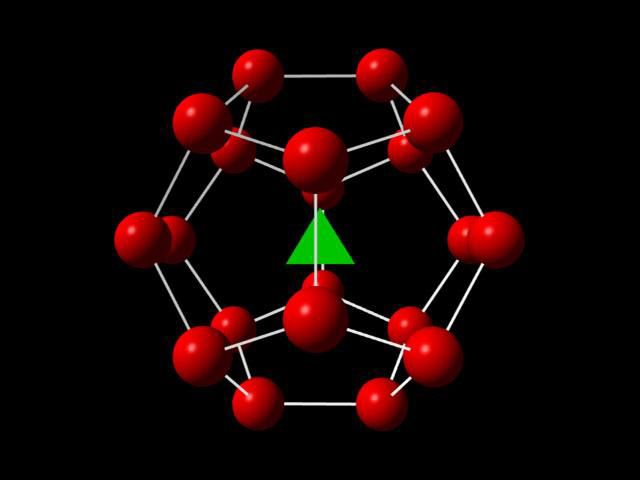 The structure of methane hydrate, with methane (green) surrounded by water (red). (Image courtesy Research Consortium for Methane Hydrate Resources in Japan.)
The structure of methane hydrate, with methane (green) surrounded by water (red). (Image courtesy Research Consortium for Methane Hydrate Resources in Japan.)
The primary reason for the difficulty of developing methane hydrates as an energy resource is the lack of an efficient means for extracting individual methane molecules from their water cages. As is clear from the graph, heating or decompression will destabilize and disassociate the hydrates, but unless this can be done over a wide area, the yield of free methane will be relatively small.
From 2001 to 2008, the first phase of Japanese methane production trials was performed on hydrates underlying terrestrial permafrost in Canada, thereby avoiding the complications of working in deep water. The initial experiments attempted hydrothermal extraction, but because the chemical reactions that occur during separation consumed the very heat needed to drive disassociation, production of methane gas stopped after only a very limited area was converted. After further study, the next round of attempts beginning in 2007 abandoned heating and successfully produced methane gas for six straight days though decompression. When the experiment ended, the technicians on site were confident that production could have been sustained for even longer. The success of the second land-based experiment highlighted decompression techniques as the more viable route to commercialization of methane hydrates.
The second reason for difficulty in production is that sub-seafloor methane hydrates are typically sandwiched between layers of soft sediment. Upon decompression, large amounts of sediment may foul operations by flowing up the well, or the methane hydrate layer may be recompacted by the pressure of the overlying sediment. Thus, during the second phase of trials from 2009 various technologies were developed to overcome these problems. Finally, in March 2013, the innovations proved successful as researchers produced methane gas for the first time from marine hydrates, removing a major obstacle to commercialization.
The Current Stage of Development
Ishii Yoshinori, University of Tokyo professor emeritus and the former director of the National Institute for Environmental Studies, has opposed this string of development programs. In the early 1990s, Japan began research and development of methane hydrates in earnest, with Ishii chairing a key research committee. This gave his later criticisms particular weight. Two decades ago, the thinking was that geothermal heating resulted in free methane below the hydrated layers, and that this gas could be easily extracted. Ishii later concluded that the existence of gas was unlikely, making extraction cost prohibitive, and that methane hydrates are fundamentally not a viable resource.
Until recently, a large number of researchers also believed similarly that extraction of methane gas was not economically feasible. However, not only has the pace of technological innovation exceeded expectations, successful production from previously cost prohibitive reserves, such as shale gas and oil, oil sands, and deep marine gas and oil, has placed methane hydrates in a more favorable light. Additionally, due to the 16-fold increase in crude oil price from 1999 to 2008, traditional wisdom regarding cost effectiveness is no longer applicable, and there certainly is potential in methane hydrates, even if production costs remain relatively high.
However, major technical hurdles remain. For example, while the recent marine trials successfully produced methane gas from hydrate, an unexpectedly large volume of sediment flow forced an early end to operations, and separation of methane was only confirmed to have occurred in a 20-meter radius around the drill pipe. Even if these technical issues are overcome, commercial production will only be possible if market price is competitive to that of shale gas and other competing reservoirs.
One more round of marine trials is scheduled for completion by 2015, followed by the final evaluation phase from 2016 to 2018. The Basic Plan on Ocean Policy introduced at the beginning of this article calls for the establishment of the technology necessary for commercialization by fiscal 2018, to be followed by study of the international situation and development of technology to allow private commercialization from 2023 to 2027.
However the above has been often misrepresented in the news as “commercial production planned for 2018,” leaving the impression that this new energy source will be available in only 5 years. Such sensational headlines were probably meant to attract attention, but in this case, it seems that journalists have failed to appreciate the developmental challenges ahead.
Still at “Level 1”
The strength of products in general, energy production methods included, can be graded according to the following levels:
Level 0: No production value
Level 1: Production scientifically possible (trial production)
Level 2: Production technically possible (preliminary production)
Level 3: Production economically viable (commercial production)
Level 4: Industry changing
Methane hydrate production is currently at Level 1. Ishii would likely say hydrates will remain forever at Level 0, but marine trial production is already moving toward Level 2. In comparison, hybrid automobiles spent an extended amount of time at Level 0 before the efforts of Toyota and Honda elevated them to Level 3. With their small market share, though, hybrids have thus far been unable to attain Level 4.
At the risk of sounding pessimistic, we must note that the idea of solving all of Japan’s energy issues with methane hydrates is simply out of the question. Before commercialization is possible, needed first is the technology for sustained production. At most, natural gas will only ever account for around 20% of Japan’s energy consumption, half that of petroleum. Due to issues regarding stable usage, compressed natural gas is not likely to replace petroleum as an automotive fuel in the near future.
Even if extensive, recoverable methane hydrate deposits exist in Japanese waters, they would only supply a portion of domestic energy needs. Therefore, it is important to calmly observe future energy developments.
(Originally written in Japanese on April 8. Top photo: an artificially formed sample of methane hydrate burned to show its energy potential. Photo courtesy Research Consortium for Methane Hydrate Resources in Japan.)
(*1) ^ On April 26 the cabinet of Prime Minister Abe Shinzō formally approved the new Ocean Policy Basic Plan.—Ed.
natural gas shale gas economy energy science methane hydrate technology Shale revolution Ishikawa Kenji resource marine strategies methan hydrate