Japan Panel OKs Medical Products Using iPS Cells
Newsfrom Japan
Society Science- English
- 日本語
- 简体字
- 繁體字
- Français
- Español
- العربية
- Русский
Tokyo, Feb. 19 (Jiji Press)--A Japanese panel of experts on Thursday gave the green light for the health minister to approve the production and sale of two regenerative medical products that use induced pluripotent stem, or iPS, cells.
The products, if approved by the minister, are expected to be the world's first of their kind.
The panel examined the products under a conditional approval system. Even if approved, additional data on the products will be collected for up to seven years.
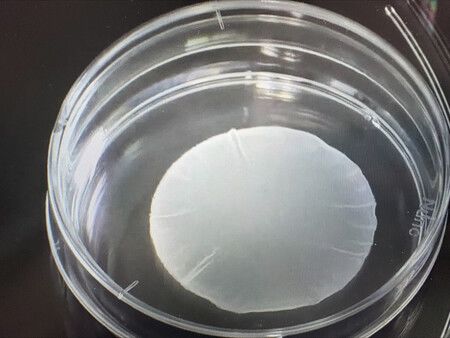
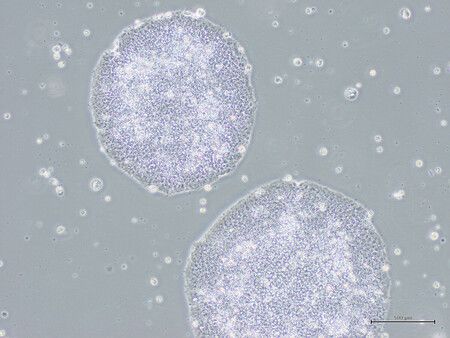
The products--cardiomyocyte patches for heart disease patients and dopaminergic neurons for Parkinson's disease patients--were developed by Cuorips Inc., a startup originating from the University of Osaka, and Sumitomo Pharma Co., respectively.
While iPS cells are made with cells taken from patients themselves or others and expected to reduce rejection reactions, ensuring long-term safety has remained a challenge.
[Copyright The Jiji Press, Ltd.]